Ocular Oximetry: A Paradigm Shift in Retinal Vein Occlusion Management?
Retinal vein occlusion (RVO) affects more than 1.1 million people in the U.S. and is considered to be an important cause of vision loss in older adults. (1)
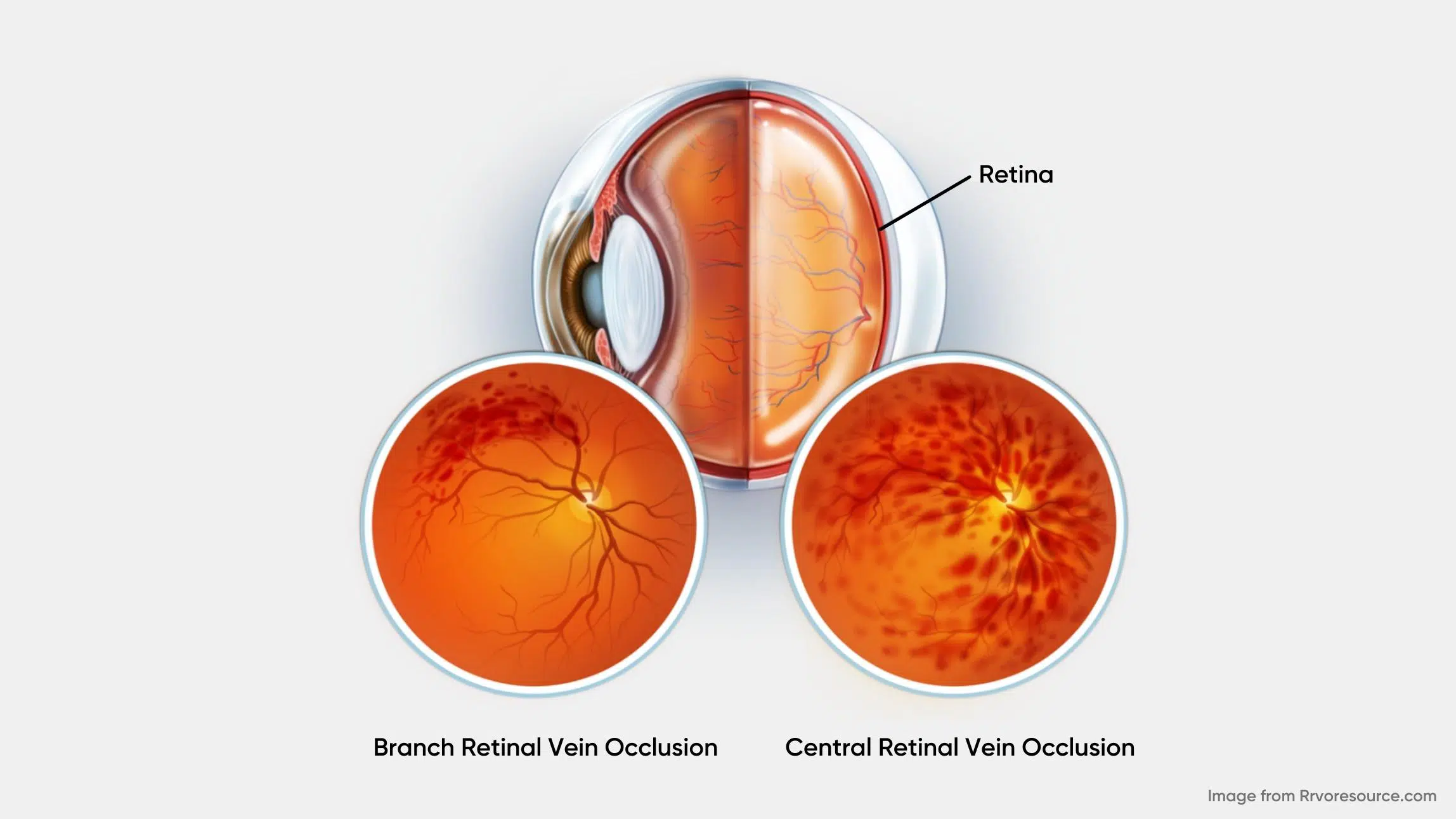
There are two types of RVO, based on the specific vasculature involved: central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO). (2) CRVO occurs when there is a blockage of the main retinal vein posterior to the optic nerve’s lamina cribrosa and is classically a result of thrombosis. CRVO can be further subdivided into the ischemic (non-perfused) and non-ischemic (perfused) category. Approximately 70% of cases are non-ischemic, characterized by relatively good visual acuity (better than 20/200), mild or no afferent pupillary defect, and mild visual changes. The remaining 30% of cases are ischemic, characterized by at least 10 disc diameters of retinal capillary nonperfusion and poor visual prognosis. (2) The resulting hypoxia can increase vascular endothelial growth factor (VEGF), inducing macular edema and iris, angle or retinal neovascularization. (3)
BRVO, on the other side, is an occlusion occurring in one of the tributaries of the central retinal vein. (2) The most common symptom of BRVO is blurry vision or vision loss in part of one eye. It can happen suddenly or become worse over several hours or days.
Pathophysiology, Epidemiology and Risk Factors
The estimated prevalence of RVO in those aged 30–89 years old in 2015 was 28 million people globally. (5) Age is a significant risk factor for the development of RVO, with 90% of patients affected being over the age of 50. (2) Other risk factors include, glaucoma, optic disc drusen, optic disc edema, hypercoagulable state, syphilis, sarcoidosis, African American race, sickle cell, human immunodeficiency virus (HIV), vasculitis, the use of certain medicines such as oral contraceptives and diuretics, abnormal platelet function, orbital disease, and seldom migraines. (2)
As thrombosis is the main cause for RVO, medical conditions leading to an increase in thrombosis-contributing elements, such as venous stasis, endothelial damage, or hypercoagulability could lead to RVO. (2) Finally, lifestyle habits such as alcohol consumption and smoking may also have a direct impact on RVO as they contribute to hypertension, diabetes mellitus, and hyperlipidemia, which are all known risk factors for retinal vascular disease. (4)
Diagnosis
The diagnosis of RVO is done primarily through a comprehensive eye examination, including pupillary evaluation and dilated fundoscopy. Once the vascular disturbance has been visualized, fundus photography can be used for patient education, documentation, and serial imaging over time. Standard optical coherence tomography (OCT) is also valuable in checking for the presence or absence of macular edema, which can alter the condition management. Fluorescein angiography (FA), a more invasive approach, can be used to determine the perfusion status of the retina and to evaluate deficiency in the amount of oxygen reaching the tissues in a qualitative manner. The advent of optical coherence tomography angiography (OCT-A) provides an alternative, non-invasive means of imaging the deep and superficial retinal capillary networks as well. In fact, it’s been found to be more effective than FA at displaying and analyzing the capillary network of the macula, as well as detecting macular edema. (6) Unfortunately, this technology is not without limitations on its own as OCT-A is subject to various artifacts such as shadowing artifacts associated with intraretinal/subretinal hemorrhage, projection artifacts of the superficial retinal vessels over the deeper retinal layers and motion artifacts. (12)
Treatment
There are many therapeutic interventions available for RVO, including laser therapy, anti-VEGF agents, steroids, and surgery. (6) By and large, anti-VEGF agents are the mainstay of treatment for RVO today.
In the quest of unveiling new hemodynamic biomarkers, great progress has been made in understanding the importance of oxygenation dysregulation in RVO.
Retinal Vessel Oximetry
During the past decade, multiple studies using traditional two-wavelength oximeters have indicated that oxygen saturation in large blood vessels is affected in RVO. In a normative study, the estimated mean (±SD) oxygen saturation of hemoglobin in healthy individuals was 92.2 ± 3.7% in arteries and 55.6 ± 6.3% in veins. (11) These values in the retinal venules of eyes affected by CRVO have been found to be significantly lower than those in fellow unaffected eyes. This is likely due to the vascular blockage causing decreased blood flow, and therefore reduced oxygen delivery to the tissue. Hypoxic tissue consumes more oxygen from the blood as vascular flow through the capillary bed is decreased. Arteriolar saturation, in contrast, is not found to significantly differ between affected eyes and fellow unaffected eyes. (8) This is consistent with the known pathophysiology of CRVO.
Furthermore, it has been demonstrated that inter-individual differences exist in retinal vessels oximetry measurements in RVO. Obstruction differs between patients, due to various compensatory mechanisms initiated by the venous occlusion. The progressive canalization of the thrombus, along with the development of collateral vessels, also contributes to this variability. (11) Research also suggests that oxygen saturation in retinal venules correlates with best-corrected visual acuity during diagnosis.
New Approach to Ocular Oximetry
While the above-mentioned studies have played a great role in establishing the link between retinal vessel oxygenation and RVO, they also contain important limitations due to the technology used to perform the measurements. Indeed, standard 2-wavelength oximetry devices provide relative measurements of oxygen saturation levels in large blood vessels only. This limitation prevents the assessment of subsurface capillary volumes on specific structures such as the retina or the optic nerve head.
To become clinically relevant, oximetry measurements must become reliable, reproducible, and accommodate various acquisition protocols, as well as static and dynamic measurements at various locations in the eye fundus. This is why Zilia has launched, for investigational use, Zilia Ocular, a fundus camera enabling the continuous measurement of oxygenation in targeted regions of the eye fundus. This novel technology paves the way to advance the non-invasive investigation of oxygenation in metabolic responses, pathophysiology, and responses to treatment.
This new approach could provide unprecedented information on the perfusion status of the retina with the ultimate goal to accurately grade the oxygen saturation in the tissue for better diagnosis, prognosis and treatment monitoring of RVO.
Summary
RVO is an important cause of vision loss in older adults, especially considering the rising rates of predisposing conditions. Traditional diagnostic tools have relied on invasive measures to generate qualitative data on retinal perfusion. In the quest of unveiling new hemodynamic biomarkers, great progress has been made in understanding the importance of oxygenation dysregulation in RVO. However, the abundance of research performed using retinal vessel oximeters has not yet translated into their deployment in clinical settings, mostly due to a lack of accuracy and versatility. Relying on the full spectrum of light, Zilia’s technology has the potential to overcome these limitations by gathering quantitative ocular oximetry data in the tissues of the eye, in a non-invasive manner, for better diagnosis, prognosis and treatment monitoring of RVO.
References
1. Klein R, Klein Be, Moss Se, Meuer Sm. The epidemiology of retinal vein occlusion: The beaver dam eye study. Trans Am Ophthalmol Soc. 98, 133-141; discussion 141-133 (2000).
2. Blair K, Czyz CN. Central Retinal Vein Occlusion. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2021. PMID: 30252241.
3. Ehlken C, Rennel E, Michels D et al. Levels of VEGF but not VEGF165b are Increased in the Vitreous of Patients With Retinal Vein Occlusion. Am J Ophthalmol. 2011;152 (2):298-303.e1. doi:10.1016/j.ajo.2011.01.040
4. Thapa R, Bajimaya S, Paudyal G et al. Prevalence, pattern and risk factors of retinal vein occlusion in an elderly population in Nepal: the Bhaktapur retina study. BMC Ophthalmol. 2017;17(1). doi:10.1186/s12886-017-0552-x
5. Song P, Xu Y, Zha M, Zhang Y, Rudan I. Global epidemiology of retinal vein occlusion: a systematic review and meta-analysis of prevalence, incidence, and risk factors. J Glob Health. 2019;9(1):010427. doi:10.7189/jogh.09.010427
6. Seknazi D, Coscas F, Sellam A, Rouimi F, Coscas G, Souied E, Glaget-Bernard A. Optical Coherence Tomography Angiography in Retinal Vein Occlusion. Retina. 2018;38(1):1562–1570. doi: 10.1097/IAE.0000000000001737
7. Schmidt-Erfurth U, Garcia-Arumi J, Gerendas B et al. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2019;242 (3):123–162. doi:10.1159/000502041
8. Hardarson SH, Stefánsson E. Oxygen saturation in central retinal vein occlusion. American journal of ophthalmology. 2010;150 (6):871-5. doi: 10.1016/j.ajo.2010.06.020
9. Krejberg Jeppesen S, Sín M, Hakon Hardarson S, Bek T. Retinal oximetry does not predict 12-month visual outcome after anti-VEGF treatment for central retinal vein occlusion: A multicentre study. Acta Ophthalmol. 2021;99(7): e1141-e1145. doi:10.1111/aos.14744
10. Yoneya, S, Saito, T, Nishiyama, Y, Deguchi, T, Takasu, M, Gil, T, & Horn, E. Retinal oxygen saturation levels in patients with central retinal vein occlusion. Ophthalmology. 2002;109 (8):1521–1526. doi: 10.1016/S0161-6420 (02)01109-0
11. Šínová I, Řehák J, Nekolová J, Jirásková NA, Haluzová P, Řeháková T, Bábková B, Hejsek L, Šín M. Correlation between ischemic index of retinal vein occlusion and oxygen saturation in retinal vessels. American Journal of Ophthalmology. 2018;188:74-80.
12. Tsai G, Banaee T, Conti FF, Singh RP. Optical Coherence Tomography Angiography in Eyes with Retinal Vein Occlusion. J Ophthalmic Vis Res. 2018;13(3):315–332. doi:10.4103/jovr.jovr_264_17
Written by the Zilia Team on January 12, 2022
More on our Blog
Zilia Partners with Kagawa University for Groundbreaking Retinal Oxygenation Study in Japan
Quebec City, Apr. 30, 2024 - Zilia, a pioneer in non-invasive ocular biomarker technologies, announces a...
Oxygen’s Complex Role in Retinopathy of Prematurity
Premature birth is a remarkable feat of medicine. However, this early entrance into the world can sometimes...
Oxidative Stress and Eye Health
Oxygen plays an important role in our universe, being the third most abundant element and the second most...
Solutions